Development of new lead composite materials for battery manufacturing and their synthesis technologies
Scientists from the Institute of Transport Systems and Technologies of the National Academy of Sciences of Ukraine have developed new composite lead materials with nano-dispersed titanium dioxide, aerosil, graphite, and carbon nanotubes. Technologies for applying a thin layer of composite electrochemical coating to the grids of lead-acid battery current collectors have been studied, and the enhanced mechanical, electrical, and corrosion properties of the obtained grids have been experimentally proven. The new lead composites will be used for the production and modification of electrode bases and active masses of lead-acid batteries to improve their performance characteristics.
Extending the service life and increasing the specific performance of batteries are key requirements of modern manufacturing and scientific research. This involves both improving battery production methods and developing new materials.
One of the approaches to modifying battery systems is the use of composite electrode materials, which can have enhanced mechanical, electrical, and corrosion properties. New composite materials can be obtained by electrodeposition of metals and dispersed non-metallic materials. The properties of composite deposits can be adjusted by selecting materials and optimizing electrolysis conditions.
By controlling the parameters of the electrolysis process and the composition of the electrolyte, composite lead materials with nano-dispersed titanium dioxide, aerosil, graphite, and carbon nanotubes have been synthesized. The features of electrocrystallization, structure, and morphology of composite deposits based on lead obtained from electrolytes containing particles of various dispersed non-metallic materials have been studied.
The use of nanoscale inclusions in the electrochemical co-deposition of metals and dispersed non-metallic materials makes it possible to obtain materials with a high specific surface area. Such composites exhibit increased electrical conductivity and improved corrosion resistance. As an example, Figure 1 presents images of lead composite deposits containing nano-dispersed titanium dioxide, aerosil, and carbon nanotube particles.
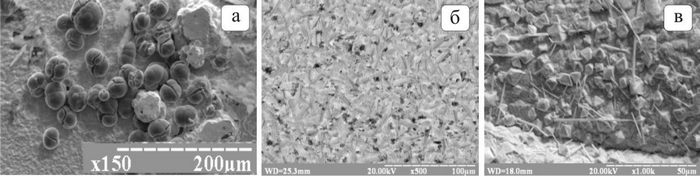
Fig. 1. Electron micrographs of the surface of lead composites with titanium dioxide (a),
aerosil (b), and carbon nanotubes (c).
Electrodeposition enables the improvement of the surface condition of the battery grid by applying a thin layer of composite electrochemical coating to its surface. Experimental studies have shown that the increased corrosion resistance of such a grid is ensured not only by the formation of an external corrosion-resistant layer but also by a more homogeneous structure of the lead material’s surface. In turn, the uniformity of the surface layer promotes even surface corrosion without localized corrosion damage with catastrophic consequences, thereby reducing the weight loss of battery grids due to corrosion. Figure 2 presents images of different areas of the lead battery grid surface before deposition (a, c) and after the deposition of the composite material layer (b, d).
The results of corrosion studies of cast lead-antimony grid samples, obtained using the gravimetric method with continuous weighing, are shown in Fig. 3. The experimental data scatter was 2-3%. As the figure demonstrates, the weight loss of traditional cast grids was approximately 4% within 1 hour of corrosion exposure. In the case of grids with a composite coating, a reduction in corrosion losses by 10-15% was observed. The fact that weight loss in coated grids was lower suggests that changes in the corrosion behavior are primarily associated with modifications in the structure of the surface layer. Specifically, the uniformity and fine-crystalline structure of the composite coating contributed to a more even corrosion process, which, in turn, led to a decrease in weight loss magnitude.
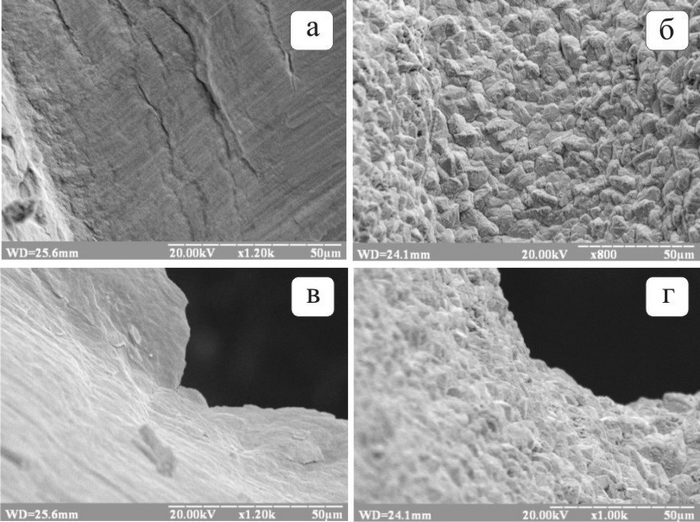
Fig. 2. Electron micrographs of the surface of the lead battery grid before deposition (a, c)
and after the deposition of the composite material layer (b, d).
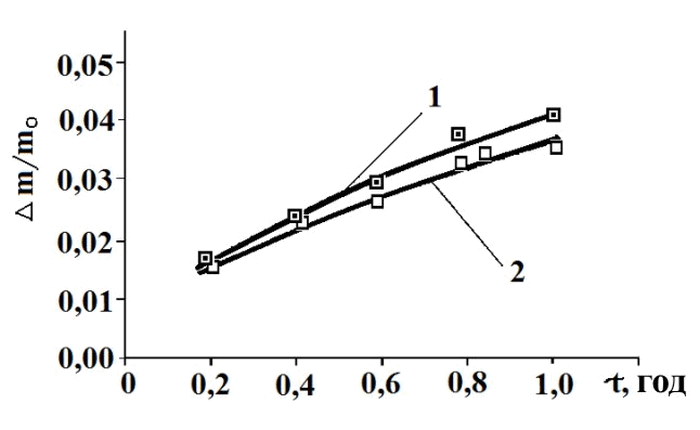
Fig. 3. Dependence of the relative weight loss of cast lead-antimony grid samples on corrosion time,
measured by the gravimetric method with continuous weighing:
1 – without composite coating; 2 – with composite coating.
Expanded metal grids made of lead-calcium alloys Pb-0.05Ca-1.1Sn and Pb-0.1Ca-0.3Sn. The results of corrosion studies of expanded metal grid samples made of lead-calcium alloys are shown in Fig. 4. As demonstrated in the figure, the weight loss of expanded metal grid samples with compositions Pb-0.1Ca-0.3Sn and Pb-0.05Ca-1.1Sn (curves 1 and 3 in Fig. 4) averaged 2% and 2.5%, respectively, after 1 hour of exposure to the corrosive environment. Meanwhile, the weight loss of the samples with composite coatings decreased to 1.5% and 2%, respectively.
Based on the obtained results, it can be assumed that electrochemical deposition of a thin layer of composite electrochemical coating onto the grid surface not only ensured the uniformity of the surface corrosion-resistant layer structure but also partially (or completely) blocked the corrosive effects of residual mechanical stresses that arise due to deformation (e.g., pitting, deep cracks).
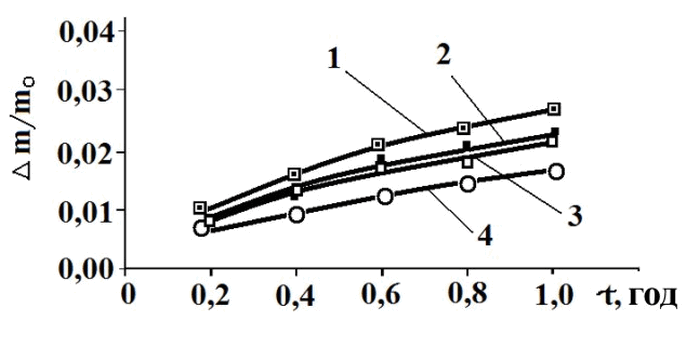
Fig. 4. Dependence of the relative weight loss of expanded metal grid samples on time, measured by the gravimetric method with continuous weighing:
Pb-0.1Ca-0.3Sn alloy without composite coating – 1, with composite coating – 2;
Pb-0.05Ca-1.1Sn alloy without composite coating – 3, with composite coating – 4.
The new lead composites will be used for the production and modification of electrode bases and active masses of lead-acid batteries to improve their performance characteristics.
